A group of researchers led by Anna-Karin Gustavsson at Rice College has developed an revolutionary imaging platform that guarantees to enhance our understanding of mobile buildings on the nanoscale. This platform, known as soTILT3D for single-objective tilted mild sheet with 3D level unfold features (PSFs), presents important developments in super-resolution microscopy, enabling quick and exact 3D imaging of a number of mobile buildings whereas the extracellular atmosphere will be managed and flexibly adjusted. The analysis was lately printed in Nature Communications.
Finding out cells on the nanoscale offers insights into the intricate mechanisms that drive mobile conduct, enabling researchers to uncover particulars which might be important for understanding well being and illness. These particulars can reveal how molecular interactions contribute to mobile features, which is vital for advancing focused therapies and understanding illness pathogenesis.
Whereas typical fluorescence microscopy has been helpful for learning mobile buildings, it has been restricted by the diffraction of sunshine, proscribing its capacity to resolve options smaller than just a few hundred nanometers. Furthermore, whereas single-molecule super-resolution microscopy has supplied groundbreaking insights into organic buildings on the nanoscale, current methods usually endure from excessive background fluorescence and sluggish imaging speeds, significantly when coping with thick samples or advanced cell aggregates. Additionally they usually lack exact, adjustable management of the pattern atmosphere.
The soTILT3D platform instantly addresses these challenges. By synergistically integrating an angled mild sheet, a nanoprinted microfluidic system and superior computational instruments, soTILT3D considerably improves imaging precision and pace, permitting for clearer visualization of how totally different mobile buildings work together on the nanoscale -; even in conventionally difficult samples.
Key improvements
The soTILT3D platform makes use of a single-objective tilted mild sheet to selectively illuminate skinny slices of a pattern, successfully enhancing the distinction by lowering background fluorescence from out-of-focus areas, particularly in thick organic samples comparable to mammalian cells.
“The sunshine sheet is fashioned utilizing the identical goal lens as used within the microscope for imaging, and it’s totally steerable, dithered to take away shadowing artifacts which might be widespread in mild sheet microscopy and angled to allow imaging all the best way right down to the coverslip,” mentioned Gustavsson, assistant professor of chemistry at Rice and corresponding writer of the examine. “This enables us to picture whole samples from high to backside with improved precision.”
The platform additionally incorporates a custom-designed microfluidic system with an embedded customizable metalized micromirror, which allows exact management over the extracellular atmosphere and permits for speedy resolution change, which is right for sequential multitarget imaging with out shade offsets whereas additionally permitting for reflection of the sunshine sheet into the pattern.
“The design and geometry of the microfluidic chip and nanoprinted insert with the micromirror will be simply tailored for varied samples and size scales, offering versatility in numerous experimental setups,” mentioned Nahima Saliba, co-first writer of the paper alongside fellow graduate scholar Gabriella Gagliano, who can also be related to the Smalley-Curl Institute and the Utilized Physics Graduate Program at Rice.
Moreover, soTILT3D leverages computational instruments comparable to deep studying for evaluation of upper fluorophore concentrations for improved imaging pace and algorithms for real-time drift correction, enabling secure, high-precision imaging over prolonged durations of time.
The platform’s PSF engineering allows 3D imaging of single molecules, whereas deep studying handles dense emitter circumstances which typical algorithms have bother with, which considerably improves the acquisition pace.”
Nahima Saliba, Rice College
SoTILT3D’s microfluidic system additionally helps automated Alternate-PAINT imaging, permitting totally different targets to be visualized sequentially with out the colour offsets widespread in multicolor approaches when imaging in-depth on the nanoscale.
Groundbreaking outcomes
The soTILT3D platform has demonstrated outstanding enhancements in imaging precision and pace. The platform’s angled mild sheet improves the signal-to-background ratio for mobile imaging by as much as six occasions in comparison with conventional epi-illumination strategies, enhancing distinction and enabling exact nanoscale localization.
“This stage of element reveals intricate points of 3D cell structure which have been historically troublesome to look at with typical approaches,” mentioned Gagliano.
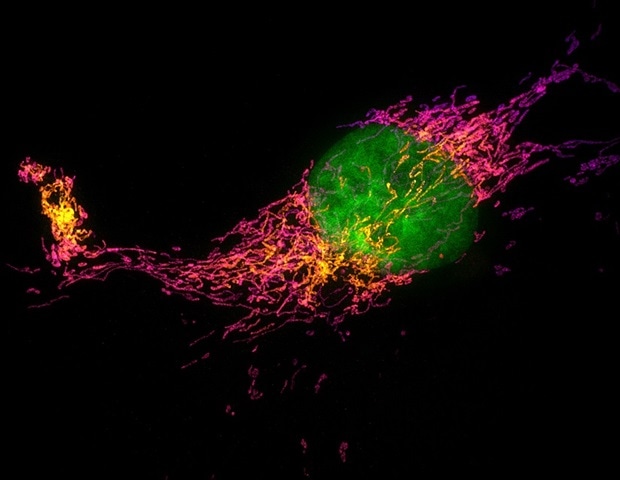
By way of pace, soTILT3D delivers a tenfold improve when mixed with excessive emitter density and deep studying evaluation, permitting researchers to seize detailed photos of advanced buildings just like the nuclear lamina, mitochondria and cell membrane proteins throughout whole cells in a fraction of the same old time. Moreover, the platform helps correct whole-cell 3D multitarget imaging, capturing the distributions of a number of proteins inside a whole cell and measuring nanoscale distances between them. Researchers can now visualize the spatial association of carefully located proteins like nuclear lamina proteins lamin B1 and lamin A/C and lamina-associated protein 2 with outstanding precision and accuracy, providing new insights into protein organizations and their position in regulating mobile operate.
Broad functions in biology and drugs
The soTILT3D platform opens new prospects for researchers throughout varied fields. Its functionality to picture advanced samples, together with stem cell aggregates, extends its utility past particular person cells. The microfluidic system’s biocompatibility makes it appropriate for live-cell imaging, permitting scientists to check mobile responses to totally different stimuli in actual time with decreased photograph injury. Its exactly managed resolution change characteristic additionally makes soTILT3D a great device for testing how drug remedies have an effect on cells in actual time.
“Our purpose with soTILT3D was to create a versatile imaging device that overcomes limitations of conventional super-resolution microscopy,” mentioned Gustavsson. “We hope these developments will improve research in biology, biophysics and biomedicine, the place intricate interactions on the nanoscale are key to understanding mobile operate in well being and pathogenesis.”
Supply:
Journal reference:
Saliba, N., et al. (2024) Entire-cell multi-target single-molecule super-resolution imaging in 3D with microfluidics and a single-objective tilted mild sheet. Nature Communications. doi.org/10.1038/s41467-024-54609-z.

